- Your cart is empty
- Continue Shopping
Pure Hypochlorous Acid: A Primer on pH and Wound Solutions
- General Knowledge
- Posted on
-
by Admin-Art

Hypochlorous Acid (HOCL): Overview
Stabilized HOCL solution has been used successfully as a wound care solution for many years. HOCL, as a preservative in these solutions, has a powerful and rapid killing effect in vitro on a broad range of microorganisms if they happen to enter the container. Additionally, it can mechanically remove biofilm formed by microbes, as well as exopolymeric matrix materials associated with biofilms.1 HOCL appears to have a favorable effect on fibroblast and keratinocyte preservation within the wound environment. These biochemical and biological factors suggest that stabilized HOCL solution may serve as an effective wound care agent.1-7 Of note, current regulatory guidelines restrict the official description of HOCL in wound solutions to that of a preservative. This is consistently true of all HOCL-containing commercial products.8
Intracellular Production of HOCL
The primary reason that HOCL at a concentration of around 300 ppm is safe in a wound cleanser is that it is naturally present in the body in similar concentrations. The role of HOCL during the human acute inflammatory response has been well documented.9-12 Innate immunity-associated cells such as neutrophils can chemically sense pathogens in their immediate environment via biochemical signaling methods, as well as by direct contact, and this sensing results in phagocytosis of the prokaryotic pathogen. In a normally functioning immune system, following attachment of the prokaryotic microbe to the eukaryotic neutrophil membrane, the microbe is engulfed into an intracellular structure known as the phagosome. A specific set of biochemical reactions is then initiated within the phagosome and results in the production of HOCL intracellularly. This leads to the rapid death and destruction of the prokaryotic organism, primarily because of cell wall destruction and essential internal protein disruption. Finally, the debris is ejected from the neutrophil along with some HOCL (Figure 1).8-10
This natural presence of HOCL in the cells of the body, as well as in the extracellular compartment, is one of the main reasons that correctly formulated pure HOCL-based wound cleansers that contain judicious levels of the acid are deemed and proven to be “natural” and benign from a cytotoxicity perspective.9-11 The level of purity is important, in that it is desirable that the cleansers contain HOCL at a purity level very close to 100%, with minimal or no presence of the related, but completely different hypochlorite anion.2,3 This is because even 5 ppm of hypochlorite has been known to interfere with cellular activity.2,3 In addition, HOCL is a far more powerful antimicrobial preservative than the hypochlorite anion.3 From all these factors taken into consideration, what an HOCL-based wound cleanser should contain is pure HOCL, and little or desirably no hypochlorite species.
Figure 1
The human inflammatory response of the neutrophil phagocytizing a bacterial pathogen with subsequent generation of HOCL.
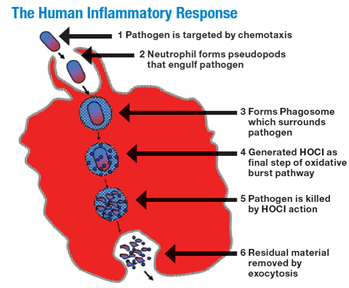
From The scientific bases for the use of hypochlorous acid to avoid pitfalls. Wounds. 2014;26(suppl):s46-s67. Image provided courtesy of Urgo Medical.13
pH Matters: Influence of pH on Wound Healing
Not all solutions that contain HOCL as a preservative are created equal. Some are formulated to remain within a pH range that is similar to that of intact skin, whereas others deliver a solution with a higher or lower pH that is hostile to the epidermal cell layers and has the potential to induce cytotoxicity.2,3 Additionally, these “higher than the desirable range” pH products will always contain some quantity of the problematic hypochlorite anion.
Of course, we know that there is an association between wound pH and the healing process. As chronic wounds heal, there is a significant decrease in wound pH from 9 to 6 or less.11,14-17 A decrease in pH has a downward impact on harmful protease activity in the wound bed. A decreasing pH has also been found to reduce the toxicity of bacterial end products (for example, ammonia), enhance control of enzyme activity, increase destruction of abnormal collagen in the wound bed, and increase angiogenesis, and decreasing pH does not negatively impact macrophage and fibroblast activity.11,14-17
Figure 2
Chlorine-based species distribution curves as a function of pH; hypochlorous acid versus hypochlorite.
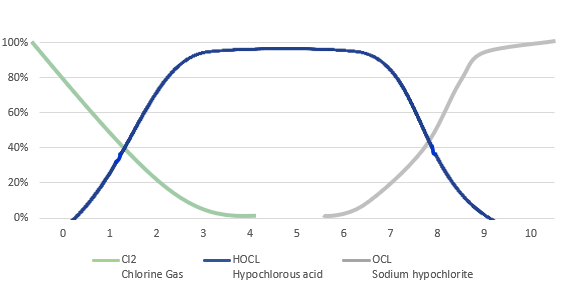
The laws of physical chemistry and a tight pH range (3.5 to 5.5) are related twin factors that ensure that HOCL is the only antimicrobial preservative present in a product formulated in the way that is shown in Figure 2,18,19 with a close to zero level of hypochlorite. This is important because the presence of the hypochlorite species, in particular, is undesirable from a cellular toxicity perspective.2,3 Moreover, the hypochlorite species will, without exception, begin to appear in any product as an impurity (to the HOCL content) if the pH of the solution is made much higher than 5.5. pH values higher than 6 have been found in some commercially available “blended” products containing HOCL.3-5 At the tightly controlled pH of 3.5 to 5.5, and the highest in the category concentration of 300 ppm, pure HOCL as available to clinicians today is completely uncontaminated with hypochlorite and therefore is harmless to fragile cells involved in wound healing.
Any shift of pH to above 6, and higher, to the alkaline range, in HOCL-containing products also could result in the reduction of microbicidal activity because of the conversion of HOCL to the less powerful hypochlorite ion.3 To compensate for this weakness in the antimicrobial preservative capacity associated with the hypochlorite species, some formulations that contain hypochlorite may need to be at high concentration, and they may require a longer microbe exposure time, both of which have a negative impact on cytotoxicity.12,13,19
Given the intellectual property protections surrounding the pH range of some pure HOCL solutions, other “blended” commercial products, as explained earlier, have to contend with the presence of some quantity of hypochlorite ions because of formulation limitations, which may introduce cellular toxicity into the wound care process. Conversely, if the pH is lower than 3, one must contend with the harmful chlorine molecule beginning to contaminate the product. With respect to the hypochlorite alone, the exact level of this undesirable species, versus the level of the desirable HOCL, depends on the pH (see Figure 2). In addition, the wound solution “blends” available to the clinician today also seem to have a lower concentration of HOCL, which is present at the highest commercially available level of 300 ppm in other pure HOCL-based commercial formulations. The concentration of HOCL likely matters in the clinical performance of the product.12
Conclusion
It is worth re-emphasizing that the “blended” and impure nature of these “outside the correct pH range” products, with respect to the only desired species, HOCL, is a consequence of their pH falling outside the desired range of 3.5 to 5.5. This specific range is uniquely associated with a purer solution of HOCL. With respect to this important ingredient, HOCL, the product purity associated with these pH 3.5 to 5.5 formulations, as opposed to other blended commercial products whose pH may fall outside this range, is related to the immutable laws of physical chemistry as represented in the pH curve presented in Figure 2.
The treatment of complex wounds is challenging both for our patients and for those of us who have the privilege of participating in their care. As physicians, nurses, and all those who engage in this field, it is incumbent upon us to understand “the what, the why, and the how” of the tools in our medical armamentarium. The biochemistry, potential for cellular toxicity, and wound pathophysiology are important factors that should be considered in the selection of any wound cleansing agent. This summary is intended to provide information that may assist the reader in selecting the most appropriate therapeutic intervention that will help to improve the care of their patients.
About the Author
Luis G. Fernández, M.D., KHS, KCOEG, FACS, FASAS, FCCP, FCCM, FICS
Professor of Surgery, Department of Surgery, Division of Trauma Surgery/Surgical Critical Care, University of Texas Health Science Center, Tyler, Texas
References
1. Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. Hypochlorous acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds. 2014;26(12):342-350.
2. Hidalgo E, Bartolome R, Dominguez C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem Biol Interact. 2002;139(3):265-282.
3. Wang L, Bassir M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I: stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5.
4. Matthews M, Quan A, Shah A, et al. Hypochlorous acid for septic abdominal processes using a unique negative pressure wound therapy system: a pilot study. Surg Sci. 2018;9:412-421.
5. Fernández LG, Matthews MR, Seal L. Intraabdominal lavage of hypochlorous acid: a new paradigm for the septic and open abdomen. Wounds. 2020;32(4):107-114
6. Armstrong DG, Bohn G, Glat P, et al. Expert recommendations for the use of hypochlorous solution: science and clinical application. Ostomy Wound Manage. 2015;61(5):S2-S19.
7. Fernández L, Ellman C, Jackson P. Initial experience using a novel reticulated open cell foam dressing with through holes during negative pressure wound therapy with instillation for management of pressure ulcers. J Trauma Treat. 2017;6:410.
8. Block SS, ed. Disinfection, Sterilization, and Preservation. Philadelphia, PA: Lea & Febiger; 2000.
9. Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187(suppl 2): S340-S345, S348.
10. Garin J, Diez R, Kieffer S, et al. The phagosome proteome: insight into phagosome functions. Cell Biol. 2001;152(1):165-180.
11. Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol. 2012;844:115-124.
14. Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007;3(3):52.
15. Shi L, Ramsay S, Ermis R, Carson D. pH in the bacteria-contaminated wound and its impact on clostridium histolyticum collagenase activity: implications for the use of collagenase wound debridement agents. J Wound Ostomy Continence Nurs. 2011;38(5):514-521.
16. Nagoba BS, Suryawanshi NM, Wadher B, Selkar S. Acidic environment and wound healing: a review, Wounds. 2015;27(1):5-11.
17. Shukla VK, Shukla D, Tiwary SK, Agrawal S, Rastogi A. Evaluation of pH measurement as a method of wound assessment. Wound Care. 2007;16(7):291-294.
18. Sharpe JR, Booth S, Kasia J, Jordan NR, Lawrence-Watt DJ, Dheansa BS. Progression of wound pH during the course of healing in burns. J Burn Care Res. 2013;34(3): e201-e208.
19. White GC. Handbook of Chlorination and Alternative Disinfectants. 4th ed. New York, NY: Wiley Interscience; 1999:215-219.
13. European Union. Risk assessment report: sodium hypochlorite. 2007:20. orats_final_rar_sodiumhypochlorite_en.pdf. Accessed September 15, 2020.
12. Non-cytotoxic wound bed preparation. http://urgomedical.us/wp-content/uploads/2016/11/Vashe-Wound-Cleansing-F…. Accessed October 29, 2020.
20. Peck B, Workeneh B, Kadikoy H, Patel SJ, Abdellatif A. Spectrum of sodium hypochlorite toxicity in man-also a concern for nephrologists. NDT Plus. 2011;4(4):231-235. doi:10.1093/ndtplus/sfr053
